Adults with overweight and obesity who do not lose enough weight on lifestyle interventions alone should receive long-term pharmacotherapy with antiobesity medications (AOM) such as semaglutide 2.4 mg, liraglutide 3.0 mg, phentermine-topiramate ER, and naltrexone-bupropion ER, according to new guidelines issued by the American Gastroenterological Association (AGA). “Each of the 4 drugs used adjunctively with lifestyle interventions is likely to result in a high proportion of patients achieving 5% and 10% [total body weight loss], which has a significant favorable effect on long-term health outcomes,” the guidelines committee writes.
The first of 9 recommendations emphasizes treating overweight/obesity as a chronic disease: “AOMs generally need to be used chronically, and the selection of the medication or intervention should be based on the clinical profile and needs of the patient, including, but not limited to, comorbidities, patients’ preferences, costs, and access to the therapy.” Semaglutide 2.4 mg with lifestyle modifications “may be prioritized over other approved AOMs for the long-term treatment of obesity for most patients” because its use has been associated with a greater percentage loss of total body weight. This agent also has glucoregulatory benefits useful in patients with type 2 diabetes, as does liraglutide 3.0 mg.
Phentermine-topiramate ER “may be preferentially used in patients with comorbid migraines,” the committee recommends based on the beneficial effects of topiramate, but “should be avoided in patients with a history of cardiovascular disease and uncontrolled hypertension.” Naltrexone-bupropion ER “may be considered for the treatment of overweight or obesity in patients who are attempting smoking cessation, and in patients with depression” but “should be avoided in patients with seizure disorders and used with caution in patients at risk of seizures.”
The AGA advises against the use of orlistat and notes the inherent problems with using phentermine for longer than 12 weeks or in patients with histories of cardiovascular disease. The guidelines make a similar recommendation for diethylpropion, which is approved by FDA for short-term use (12 weeks). The panel identified the “use of Gelesis100 oral superabsorbent hydrogel as a knowledge gap” and recommended use of this product “only in the context of a clinical trial.”
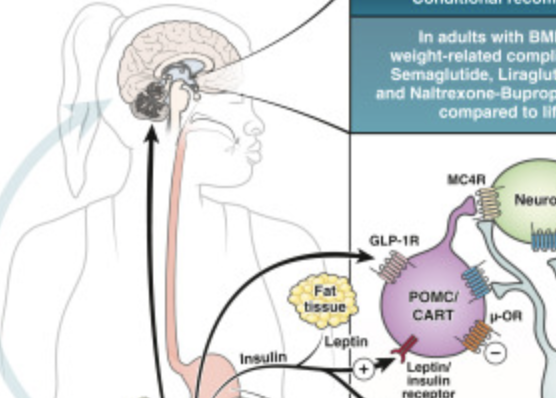
The AGA guidelines were published in conjunction with a clinical decision support tool for adults with obesity and a “Spotlight” diagram illustrating how, where, and why the medications work for this chronic disease.