With the federal public health emergency ending on May 11, the U.S. Department of Health and Human Services has released a “transition roadmap” that discusses which pandemic-related changes will remain and which will expire. Pharmacists are affected by these decisions, some of which expanded authority to administer vaccines of all types to patients across the lifespan, perform in-pharmacy COVID-19 tests, and order and administer certain COVID-19 therapeutic products such as ritonivir-boosted nirmatrelvir and casirivimab/imdevimab. Out-of-pocket expenses will change for some treatments and services, HHS said, but many of the rules and authorizations will remain in place: “FDA’s EUAs for COVID-19 products (including tests, vaccines, and treatments) will not be affected. The ending of the COVID-19 PHE will not affect the FDA’s ability to authorize various products, including tests, treatments, or vaccines for emergency use. Existing EUAs for COVID-19 products will remain in effect under Section 564 of the Federal Food, Drug, and Cosmetic Act, and the agency may continue to issue new EUAs going forward when criteria for issuance are met.”
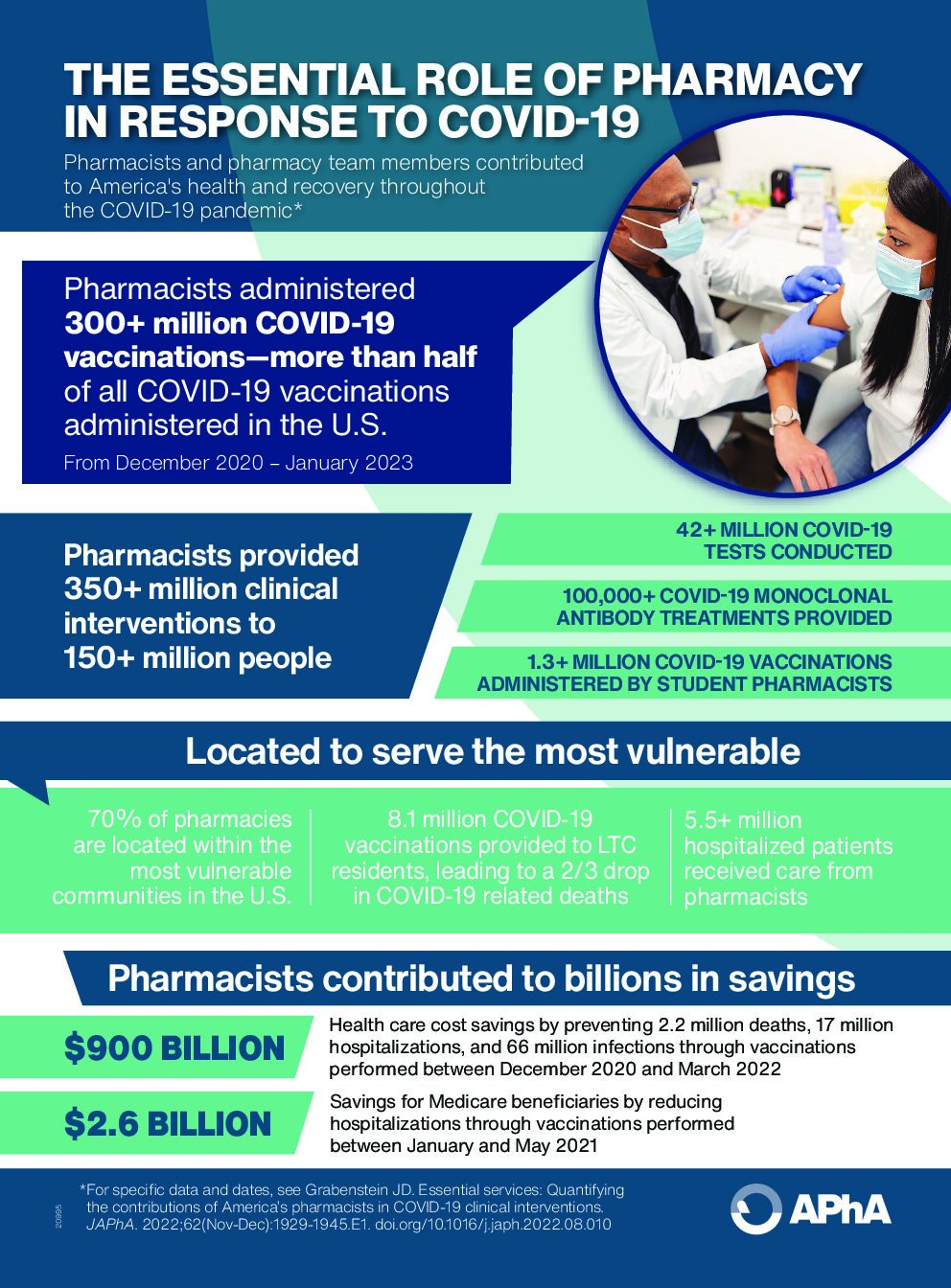
“Pharmacists and their teammates contributed to America’s health and recovery during the COVID-19 pandemic by providing >350 million clinical interventions to >150 million people in the form of testing, parenteral antibodies, vaccinations, antiviral therapies, and inpatient care,” John D. Grabenstein wrote in a recent article in the Journal of the American Pharmacists Association. “Using conservative estimates, pandemic interventions by pharmacists and teammates averted >1 million deaths, >8 million hospitalizations, and $450 billion in health care costs.” APhA released an infographic summarizing key data from the study.